Our Vision
Transform obstructive sleep apnea (OSA) diagnosis and management through the development of an AI-powered point-of-care diagnostic platform that enables more healthcare providers in more settings to provide convenient, cost-effective, accurate, and actionable results in real-time.
Our Mission
Our mission is to empower healthcare providers with new diagnostic capabilities at the point of care.
Our Team
Perry Mansfield, MD Founder, Executive Chairman, and Chief Medical Officer

Dr. Mansfield is Director of Skull Base Surgery/Head and Neck Oncology and Fellowship Director at Senta Clinic. Dr. Mansfield has extensive experience in Sleep apnea surgical management in complex airway surgeries. He has operated on over 12,000 patients and has innovated many techniques. He has presented extensively nationally and internationally and is a regular invited expert guest lecturer. He has been Chairman of four Head and Neck Surgery programs and currently serves as Director Scripps Prebys Cancer Center; Advanced Head and Neck, Cranial/Orbital/ Maxillofacial Surgery Program.
Chris Studer President

Mr. Studer is a 4X entrepreneur with a track-record of delivering product and service innovation along with building revenue streams and operating income. He has built and led startup teams in technology, education, financial services, and life sciences - including the last 11 years in digital health and diagnostics. Mr. Studer's prior companies developed first-of-its- kind technology platforms that achieved significant commercial success reaching $200M in revenue.
Hooman Sedghamiz, MS Head of AI/ML Development

Mr. Sedghamiz has a deep-seated expertise in AI and ML applied to life sciences. He has led teams and projects that enhance medical products,including implantable and wearable devices. Mr. Sedghamiz has led data science projects from inception through to commercialization, managed cross-functional teams, and bridged the gap between medical and healthcareapplications through MLOps, LlmOps, and DL product management.
Ross Mandeville, MD Director, Clinical Development

Dr. Mandeville is a clinician-scientist trained inneuromuscular neurology, neurophysiology, and clinical research, with experience across the translational spectrum from bench to bedside. He transitioned from clinical academic practice to a productive and translational research path that intersects the innovation and application of state-of-the-art quantitative neurophysiological and neuromuscular imaging approaches.
Board & Advisors
S. Wayne Kay, MBA Independent Director

Mr. Kay is a 40-year veteran of clinical diagnostic/medical device industry as an entrepreneur/CEO - including Glaxo SmithKline Diagnostics, Quidel, Response Biomedical and CardioNexus. Additionally, Mr. Kay served as the Interim CEO of two early-stage medical diagnostic companies spun out of the California Institute of Technology.
Atul Malhotra, MD Advisor/Consultant

Dr. Malhotra provides guidance and expertise for our scientific and clinical activities pending the hiring of Chief Scientific Officer. Dr. Malhotra is Vice Chair of Medicine for Research and Endowed Professor in Respiratory Medicine and Research Chief in Pulmonary, Critical Care, Sleep Medicine and Physiology at the University of California, San Diego (UCSD). He runs a large NIH funded laboratory with over 700 peer-reviewed publications (>425 original) since 2000. field.
Shamim Nemati, PhD Advisor/Consultant

Dr. Nemati serves as consultant and advisor focused on the development of our AI-powered diagnostic platform. Dr. Nemati is Director of Predictive Health Analytics and Associate Professor of Biomedical Informatics at UCSD. Dr. Nemati is an internationally acknowledged expert in clinical predictive modeling. He obtained his Ph.D. degree in Electrical Engineering and Computer Science from MIT in 2013, followed by two years of postdoctoral fellowship at Harvard School of Engineering and Applied Sciences.
Technology
PMI is pioneering the use of electromyography (EMG) as the central component in the first and only AI-powered multi-modal point-of-care sleep apnea diagnostic test (AIMS-Dx). Our proprietary end-to-end platform integrates two innovations developed by PMI:
Transmembranous EMG (tmEMG) Probe
First-of-its-kind technology uses non-invasive EMG sensors and instruments to assess neuromuscular function in membranous and sensitive anatomical locations by recording diagnostic-quality EMG signals from muscles. The first and only validated membranous electrode-based EMG device with diagnostic equivalence to the gold standard intramuscular needle electrode.
EMGNet
First-of-its-kind deep learning model and algorithm that uses upper airway muscle EMG data acquired using the tmEMG probe along with other clinical data elements such as images (photos and video) and EHR data to automatically detect OSA and identify multimodal biomarkers at the point-of-care for use in the diagnosis and management of OSA.
Clinical Validation of tmEMG
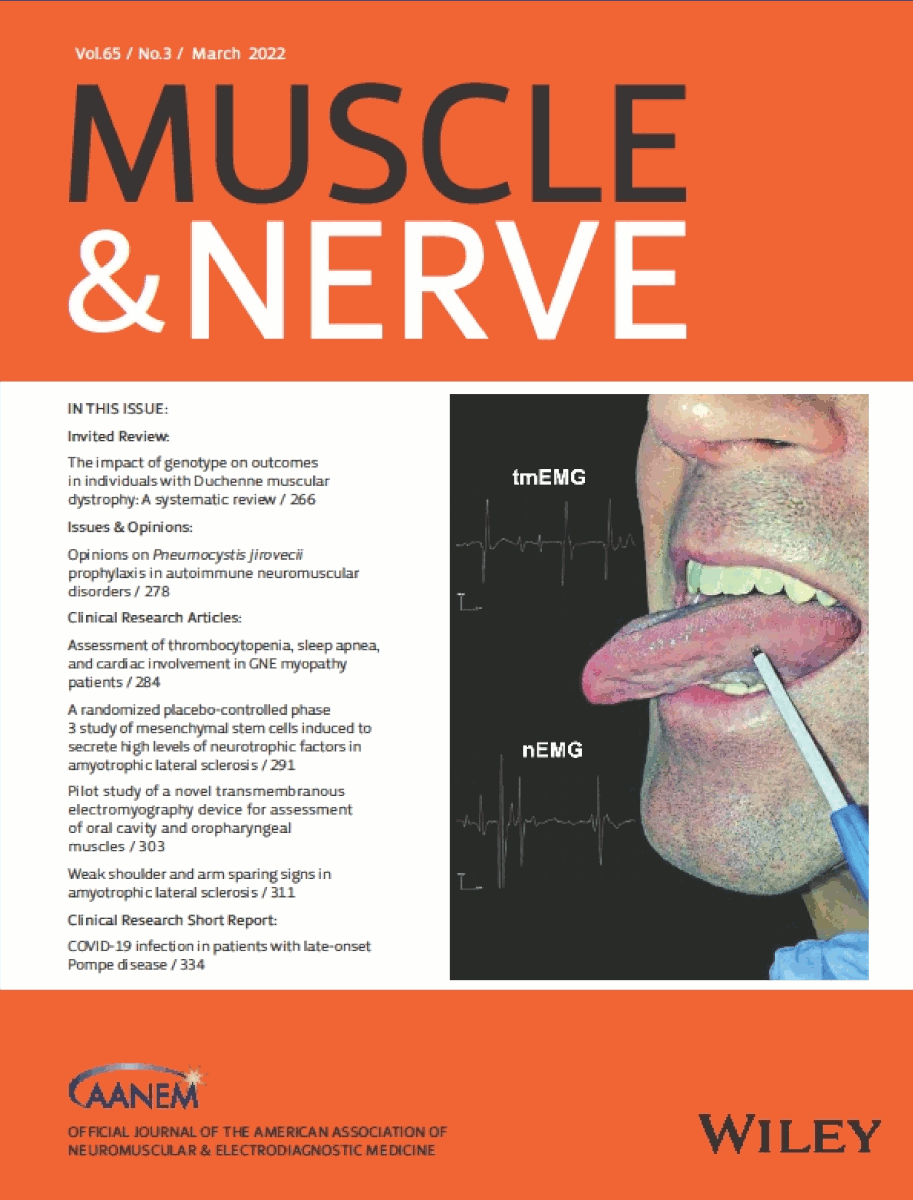

- Clinical trial published in Muscle & Nerve, March 2022.
- PMI study chosen as the cover article for the March 2022 issue.
- https://doi.org/10.1002/mus.27479
Deep Learning Enhanced Transmembranous Electromyography in the Diagnosis of Sleep Apnea

- Clinical trial published in BMC Neuroscience December 2024.
- First-ever clinical study diagnosing OSA with EMG that showed 82% accuracy.
- https://doi.org/10.1186/s12868-024-00913-9
OSA Neuromuscular Function White Paper

White paper co-authored by KOL panel members published in Frontiers in Sleep demonstrates how our technology could change the diagnostic paradigm in OSA and improve patient care and outcomes.
